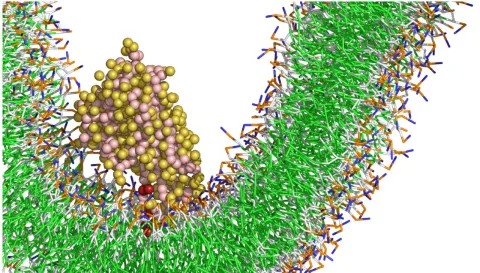
Mitochondria not only serve as the cell’s powerhouses but also play a vital role in producing key lipids, including cardiolipin—a molecule essential for mitochondrial function. Our project focuses on how specific proteins, particularly the Ups1/Mdm35 complex, transport lipid precursors across mitochondrial membranes. These lipids are necessary for synthesizing cardiolipin, and disruptions in this process are linked to diseases such as Barth syndrome, Parkinson’s, and Alzheimer’s.
Despite knowing the proteins involved, many molecular details remain unclear—especially how membrane properties like curvature, charge, and lipid composition influence the efficiency and direction of lipid transport. The project combines biochemistry, biophysics, molecular simulations, and microscopy to uncover how these factors regulate Ups1’s function. Additionally, researchers aim to understand how Ups1 forms higher-order structures that might help bring membranes closer together, potentially accelerating lipid transfer.
By clarifying these fundamental processes, the research provides insight into the molecular origins of lipid-related mitochondrial diseases. It also contributes to a broader understanding of how membrane dynamics shape cellular life—uniting molecular mechanism with functional relevance in health and disease.
