Cells remodel their membranes to generate defined organelle shapes and ensure proper organelle function. The endoplasmic reticulum (ER) is a multifunctional organelle with a particularly elaborate architecture. Moreover, depending on cell type and physiological situation, the ER can adopt a variety of morphologies to support diverse cellular needs. Understanding how distinct ER morphologies arise and how they enable specific ER functions is a long-standing challenge.
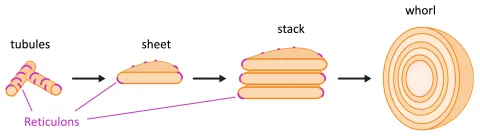
Recent progress has defined mechanisms through which cells generate the basic structural elements of the ER, namely tubules and sheets. However, it remains unclear how cells control ER remodelling and how they form more complex ER structures, such as stacks and whorls. In this project, we investigate dynamic ER remodelling in yeast and test the hypothesis that stress-induced clustering of ER-shaping Reticulon proteins helps determine ER shape and volume. In addition, we explore how ER stacks and whorls are formed. We are characterising the structure and composition of stacks and whorls, and we want to uncover the molecular machinery for generating these specialised organelle subdomains.
The aims of this project reflect our overall goal to identify the machinery for ER morphogenesis and uncover how it is regulated. This knowledge will be important for understanding the relationship between ER form and function. In addition, it may allow to tackle common human diseases associated with disrupted ER function, for example fatty liver disease and diabetes.
