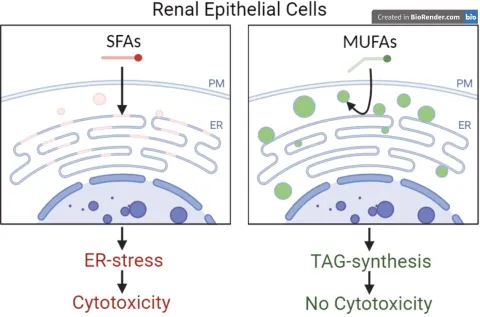
Proximal tubular cells (PTCs) of the kidney are specialized for protein and lipid uptake. Increased uptake can occur in disease conditions and may lead to proteo- and lipotoxic effects. Our previous work has shown that the uptake of albumin-bound saturated fatty acids such as palmitic acid (PA) is particularly toxic to PTCs. We found that it impairs the formation of lipid droplets (LD) and increases phospholipid saturation and, thus, membrane rigidity in the endoplasmic reticulum (ER). We and others also identified the ER-resident E3 ubiquitin ligase RNF145 as a putative sensor for membrane fluidity whose knockout protects against PA-mediated lipotoxicity. As such, RNF145 appears to be a promising target for the treatment of lipotoxic stress, for example, in diabetic kidney disease.
By combining acute and chronic RNF145 knockout and overexpression with high-resolution imaging techniques in PTCs, we will now study the effect of membrane fluidity on the structure of the ER and its ability to undergo morphological and topological transformation. In particular, we will focus on the formation of LDs, involving progressive deformation of the cytoplasmic leaflet of the ER membrane through the accumulation of neutral lipids. Finally, we will investigate the impact of RNF145 deficiency in diabetic and ischemic mouse models in which kidney disease is driven by lipotoxicity. Renal phenotyping will include detailed ER and LD analyses as well as spatial lipidomic measurements. Altogether, this project aims to understand how membrane fluidity is controlled by ER-associated degradation and how it impacts ER morphology and LD formation. In the long term, we will apply the obtained knowledge to develop new therapeutic strategies to treat kidney disease.
