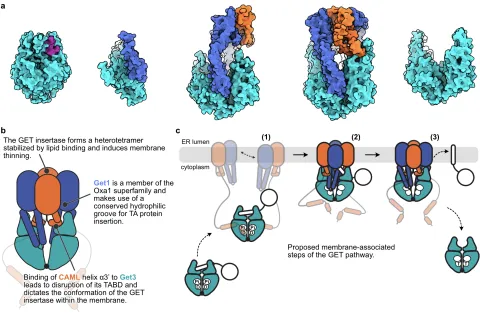
The ER membrane is the major hub of eukaryotic membrane protein insertion and might thus serve as a model membrane for deformation and sorting studies. Tail-anchored (TA) proteins are chaperoned and targeted to the ER membrane by the guided entry of tail-anchored proteins (GET) pathway. Membrane integration is mediated by an insertase consisting of two integral membrane proteins, Get1 and Get2, that operate together with the Get3 chaperone. Our cryo-EM structures of human and fungal GET insertase complexes revealed a striking structural homology of Get1 with members of the Oxa1 superfamily of protein insertases, suggesting a common mechanism of membrane insertion.
These structures serve as the starting point to investigate the structural and functional implications of lipid-protein interactions for TA protein insertion and membrane morphology at the ER membrane.
Structural studies using cryo-EM single particle analyses are combined with TA insertion studies and multiscale molecular dynamics simulations to uncover the dynamic interplay between the GET machinery, membrane lipids, and TA substrates. Overall, this project aims to understand the unifying principles and specific adaptations of membrane remodelling by the GET insertase and their impact on the fundamental process of membrane protein insertion.
