The boundary of the largest cellular compartment, the nucleus, is composed of a double membrane, which is perforated by large proteinaceous transport channels, the nuclear pore complexes (NPCs). Nuclear pores form an evolutionarily conserved nanodomain spanning the double membrane by connecting the inner and the outer nuclear membrane in a unique topology that stabilizes both negative and positive membrane curvature. Insertion of new nuclear pores requires deformation of the inner nuclear membrane, which results in the creation of a new fusion pore that is then stabilized by nuclear pore proteins. The process of pore complex insertion involves bending and fusion of the nuclear membranes and is essential for nucleocytoplasmic transport, cell cycle regulation and nuclear integrity. However, the mechanism of this asymmetric membrane deformation between two parallel membranes and the forces that drive the dynamic membrane remodeling remain enigmatic. Resolving this knowledge gap is crucial for understanding NPC-related diseases and the evolutionary origin of the eukaryotic nucleus.
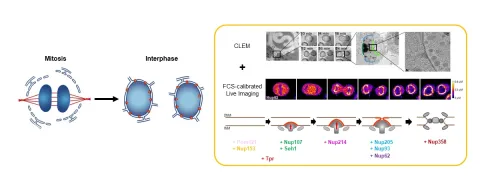
In our past work, we have used advanced light and electron microscopy to characterize the order in which nuclear pore proteins assemble and the morphological changes of the nuclear membrane they induce. We could show that pore insertion proceeds by an extrusion of the inner nuclear membrane, followed by fusion with the outer membrane. More recently, by integrating electron tomography with FCS-calibrated quantitative imaging, we demonstrated that thousands of new NPCs form within 45 minutes in the growing nucleus of living cells. We could show that pore formation is a directional process, which occurs in a well-defined molecular order with peripheral nucleoporins assembling first, followed by more centrally positioned components. However, how the assembling nucleoporins deform the inner nuclear membrane and ensure that it fuses in a controlled manner with the outer nuclear membrane without rupturing the nuclear boundary remains largely unknown.
By taking an interdisciplinary approach combining acute molecule perturbations and lipidomic analysis, as well as super-resolution and correlative cryo-electron microscopy, our goal in this project is to unravel the structure and molecular mechanism of membrane deformation during nuclear pore assembly. This work will not only provide fresh insight into the pathogenesis of NPC-related diseases but also shed light on important fundamental questions of how the complex endomembrane system of present eukaryotes may have evolved during evolution.
